Prof. Dr. med. Dietrich Tönnis
Sammlung wissenschaftlicher Arbeiten und Vorträge zur Orthopädie
Uni- and bicompartmental arthroplasties with minimal resection of the tibial subchondral bone plate.
Long term results related to various factors
© Prof. Dr. med. Dietrich Tönnis, K. J. Gerstmann, Dr. A. Heinecke - Investigations performed at the Orthopaedic Department of the Klinikum Dortmund
By clicking on a figure an enlarged version of the figure will appear. At the end of the page you will find a PDF version of the paper.
ABSTRACT
Background
The principle of the unicompartmental knee prosthesis of Tönnis was to use a thinner polyethylene tibial plateau and place it on a good or fair tibial bone surface cemented and without metal backing. The hypothesis was that the rate of loosenings should be less on the long term and revisions could be performed easily.
Material
A total of 424 unicompartmental knee arthroplasties were implanted between 1974 and 1984, and 359 of the prostheses (85%) - 39 of the Marmor type, 315 of the Tönnis type and 5 combinations -- were reexamined after periods of 1-11 years. 227 had been implanted medially, 32 laterally and 100 in both compartments of the knee.
Results
The survival rate at 11 years, when loosened components were added to revised, was 82%. The revision rate of the early Marmor prosthesis was 14.6%, and that of the Tönnis prosthesis was 6.3% (31.3% in rheumatoid patients). There were only minor differences in the results with medial, lateral and bicompartmental prostheses. Due to the sparing tibial resection, it was possible to exchange the plateau component in nearly all cases.
39.7% of the joints were free of pain. There was occasional slight pain in 37.6%, moderate pain in 13.1%, usually at the start of walking, and severe pain in only 11.4%. Normal walking was unlimited in 46.6% of the joints. In the remaining joints, walking limitation was often due to pain in other joints.
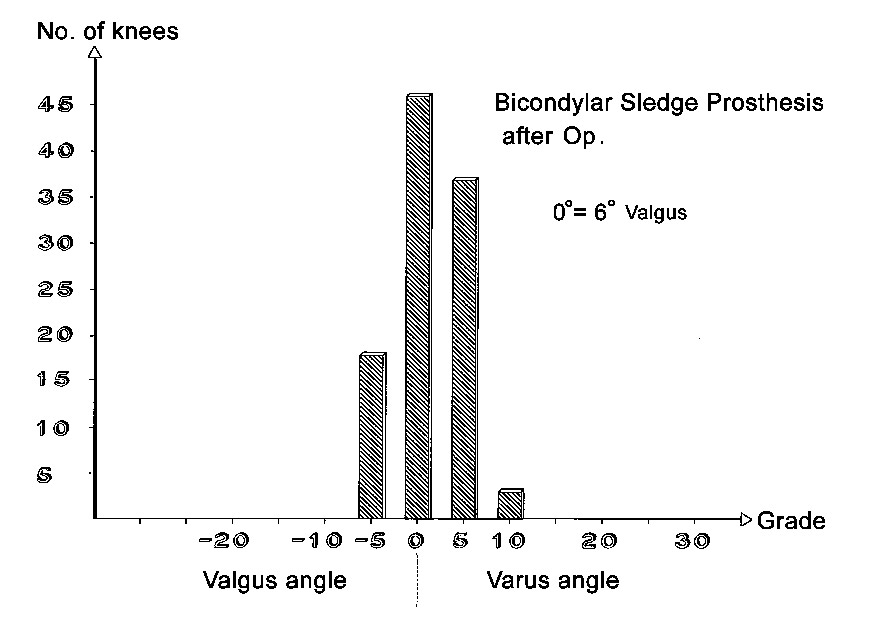
Better varus correction was achieved with bilateral unicompartmental prostheses. A position of 1° valgus = 5° varus relative to the normal 6° valgus was still present to a moderate degree because of tight medial collateral ligaments. But loosening and revision rised only where there was 7° and more varus deviation from the normal 6° of valgus (34%).
Conclusion
The revision rate for the tibial component was significantly increased only with the polyethylene plateau of 4 mm thickness (24%). The 5--7mm and 8--9mm thicknesses had equal revision rates (11-16%). The confidence limit was 0.65. Today a thickness of 8-9 mm is commonly recommended by others.
Clinical relevance
This paper is intended as an contribution to the discussion of whether it is safer in the long term to preserve the subchondral bone plate of the tibia and use thinner components designed in one-millimeter thickness increments. It also stresses the advantages of the unicompartmental sledge prostheses.
INTRODUCTION
It is only in the last 15 to 20 years that endoprosthetic knee arthroplasty has become a widely accepted treatment modality for osteoarthritic knees. Current options are the use of constrained hinged prostheses, unconstrained prostheses that completely cover the condylar bearing surfaces on the femoral side and the opposing tibial plateau, and unicompartmental resurfacing prostheses, known also as sled (or sledge) prostheses (Engelbrecht 1971). The latter type leaves the collateral and cruciate ligaments intact, thereby preserving the basic and the sensomotoric physiology of the knee joint. The runner of the sled prosthesis is cemented to the condyle, and a half-moon-shaped plateau component is cemented to the upper tibia. The sled type of prosthesis is the subject of this paper. The new mobile-bearing knee implants are not discussed.
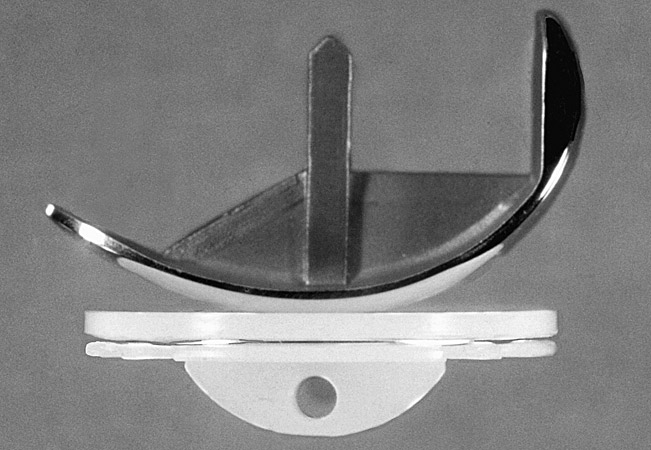
We first introduced the Marmor modular knee prosthesis in 1974 (Marmor 1973, 1984) (Fig. 1)
 (Fig. 1) Marmor unicompartmental modular knee viewed from the side. There was no anchoring tab under the polyethylene tibial compartment.
(Fig. 1) Marmor unicompartmental modular knee viewed from the side. There was no anchoring tab under the polyethylene tibial compartment.
The polyethylene plateau was originally designed as an inlay prosthesis, but soon we began placing the component onto the leveled subchondral bone of the tibial plateau, since that area is naturally structured to bear pressure while the underlying cancellous bone is not. To minimize the extent of bone resection on both sides of the joint space, we worked with the firm Waldemar Link of Hamburg to develop a sled prosthesis (Tönnis, 1979) with low-profile metallic runners that would be combined with a thin tibial polyethylene component stabilized with an anchoring tab (Figs. 2-4). Thus, our implantation technique differs fundamentally from other techniques that involve the use of an 8- or 9-mm-thick tibial component or metal backing that requires opening the area of the trabecular and cancellous bone. A key question is whether tibial components implanted in this way have a better long-term survival or whether the cancellous bone after larger resections can adapt and strengthen to the same extent.
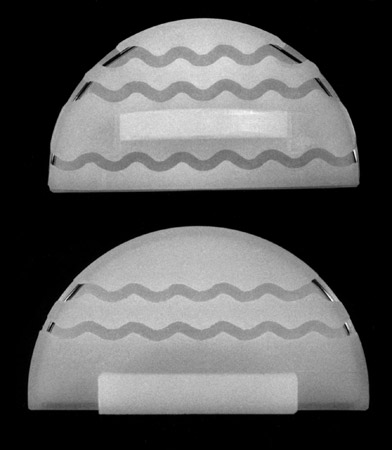 (Fig. 3) Tibial components viewed from below. Model 1 had the anchoring tab on the medial edge, model 2 has the tab in the center.
(Fig. 3) Tibial components viewed from below. Model 1 had the anchoring tab on the medial edge, model 2 has the tab in the center.
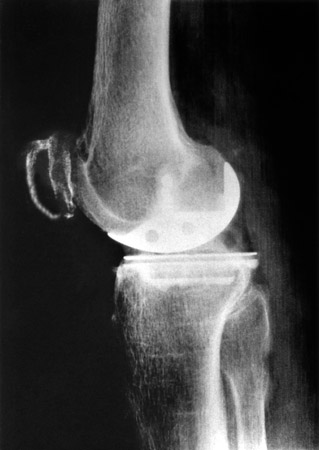 (Fig. 4) Lateral view of the arthroplasty. The superficial portion of the posterior condyle was removed with a flat chisel, coaxial to the femoral shaft, at a height of 10-12mm, or as much as necessary for sufficient flexion.
(Fig. 4) Lateral view of the arthroplasty. The superficial portion of the posterior condyle was removed with a flat chisel, coaxial to the femoral shaft, at a height of 10-12mm, or as much as necessary for sufficient flexion.
The Structures of the Upper Tibia and their Stress Tolerance
In addressing the question of how much of the load-bearing layers of the upper tibia must be preserved so that a unicompartmental plateau component will not loosen or deform, or conversely how much bone can be resected when a thicker prosthesis is used, we should briefly review our current knowledge on the anatomy of the tibial condyles.
The uppermost layer is formed by cartilage, which is thickest where the medial edges of the menisci leave the articular surface exposed (Noble, Alexander 1985). The cartilage underneath the menisci is thin. Below the cartilaginous surface is the subchondral bone plate, which in turn is supported from below by trabecular pillars (Noble, Alexander 1985). These pillars are buttressed distally by the lateral cortex (metaphyseal shell) of the upper tibia in the area where the tibial metaphysis tapers toward the shaft.
Bone density is normally lowest beneath the outer, thickest portion of the meniscus (Goldstein et al. 1983, Hvid et al. 1983, Hvid, Hansen 1985, Hvid et al. 1985, Noble, Alexander 1985). Towards its inner edge, the bone density increases and reaches its maximum a little medial to that edge. It decreases again toward the intercondylar eminence. As a result, the periphery of the upper tibia contributes little to load bearing (Finlay et al. 1989). The zone of maximum load bearing is located at the center of the medial and lateral plateaus, extending somewhat more anteriorly on the medial side and somewhat more posteriorly on the lateral side. If the menisci are damaged or removed (Bourme et al.1984) and especially if osteoarthritis is present, the bone density increases, showing an even greater increase in response to genu varum or valgum (Finlay et al.1989, Hvid, Hansen 1985, Zysset 1994).
Vasu et al. (1986) have evaluated the different material regions or layers of the tibial condyles and measured different properties. The highest strength is found in region 2, the subchondral and upper trabecular bone region. But immediately below in region 4 or 5, the elastic modulus drops from 5000 to 440 or 100, the Poisson ratio from 0.31 to 0.21 or 0.18, and the yield strength (in MPa) from 71.3 to 14.0.
Similar observations have been made by other authors. Harada et al.(1988) used indentation tests to determine the ultimate strength of the proximal tibia. Measurements were made at the subchondral bone surface and on transverse planes up to 25 mm below the surface. The medial condyles were stronger than the lateral condyles, and in both cases bone strength decreased abruptly with distance from the surface, especially over the first 5 mm.
Hvid et al. (1986) performed thin-needle axial penetration tests and axial compression tests. The penetration strength at five successive 2 mm levels beneath the resected subchondral surface decreased significantly, except for the posterolateral side initially, and then tended to level off. The reduction was most pronounced at the center of the condyles. The curve decreased rapidly after 2 mm to 5 mm and then more gradually.
Little et al. (1986) plotted the load sharing between the cancellous bone and the cylindrical cortical bone for the first 40 mm distally from the tibial eminence.
These studies show that bone strength declines significantly starting at a distance of 2 mm from the uppermost bony layers of the upper tibia. Odgaard et al. (1989) point out, however, that conventional stiffness and strain measurements are unreliable in the distal, intermediate, and proximal third of the cylindrical trabecular bone area due to a lack of trabecular constraint at the end surfaces. As a result, conventional measurements tend to underestimate failure strains.
Using the finite-element method to measure stress distribution in the tibial plateau, (Hoffmann 1995, Kilgus et al.1991) found that a plateau thickness of 4 mm generally was not adequate to withstand a test load of 1500 N and that a material thickness of at least 6--8 mm was required. Kilgus et al. (1991) had already reached the same conclusion for prostheses with nonconforming contact surfaces. Bartel et al. (1986) suggested a material thickness of 8 mm or preferably 8-10 mm. Today this thickness is considered the standard for nonconforming prostheses. In this regard, we feel that it is worthwhile to review our own study results from previous years. It probably is of importance that our tibial polyethylene plateau had finally a supporting anchoring tab in the center (Fig. 3).
METHODS
Operative Technique
Medial surgical approach
The knee joint is exposed through a medial parapatellar incision that is carried through the skin and the short medial tendinous attachments of the vastus medialis at the proximal patella. Then the incision extends 3-4 cm upward at the border of the rectus femoris tendon. We help preserve the vastus medialis by not separating it from underlying tissues or overstretching it when retracting the patella. For a bicompartmental arthroplasty, we can osteotomize the tibial tuberosity for better vision, removing a segment 5-6 cm long, 2.5 cm wide, and no less than 6-8 mm thick.
Later we tentatively fix the segment with Kirschner wires and reattach it with 2 Blauth flat-head cancellous bone screws. It should be reattached at a moderate pressure to avoid pressure necrosis. The infrapatellar fat pad is dissected slightly from the bone, but should not be removed because it contains numerous proprioceptive endings. The lateral parapatellar approach is similar.
Preparation of the femoral condyles
The knee is placed in 90° flexion and the foot immobilized with a sandbag. After the meniscus is removed, the superficial portion of the posterior femoral condyle is removed with a flat Lexer chisel, which is directed coaxial to the femoral shaft. The thickness of the osteotomy is 10--12 mm, or slightly more in a large knee joint (Fig. 4). Unless a sufficient thickness is removed, there will be too much ligamentous tension in the flexed knee, making flexion difficult and painful. A template and drill guide is then used to check whether some bone needs to be removed from the condylar surface in addition to cartilage. The prosthesis should seat against the subchondral bone surface. The tip of the runner should be sunk to the depth of the cartilage layer, if present. The runner is directed in the sagittal plane with its bearing surface at a 90° angle to the tibial axis. A 7 mm hole is drilled 40 mm deep to accommodate the stem of the prosthesis. An oscillating saw is then used to cut a narrow slot for the short, sagittal anchoring membrane of the stem. This makes it sufficient to use a single anchoring stem.
Preparation of the tibial plateau
The medial or lateral surface of the tibial plateau is resected at right angles to the tibial axis, giving the cut surface a 5° downslope from anterior to posterior to facilitate knee flexion. For this purpose we used a special drill guide placed against the long anterior tibial margin (Fig. 5). The top of the guide is fitted with a transverse plate that has a 5° posterior downslope and multiple openings for 2 mm Kirschner wires. Two of the wires, spaced 1.5-2 cm apart, are drilled in so that they are just beneath the plateau surface or barely exposed. These K-wires serve as guidewires for the oscillating saw, providing a resection that largely spares the subchondral bone plate.
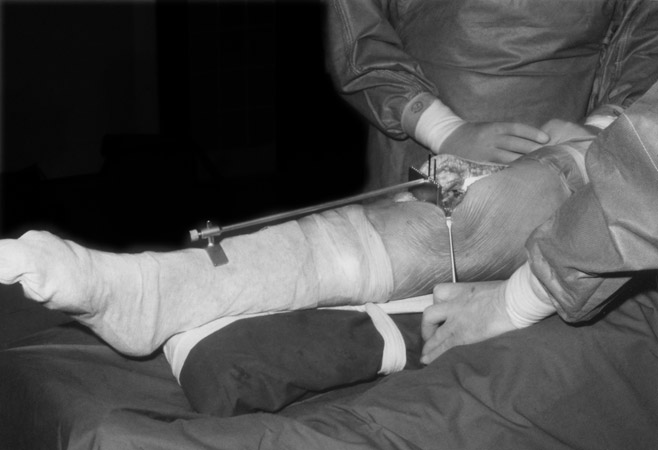 (Fig. 5) The surface of the medial or lateral tibial plateau is cut - level and perpendicular to the tibial axis. The resection spares as much of the subchondral bone plate as possible. Two Kirschner wires are drilled in to keep the saw on the desired superficial plane. A template for the wires was used as shown here.
(Fig. 5) The surface of the medial or lateral tibial plateau is cut - level and perpendicular to the tibial axis. The resection spares as much of the subchondral bone plate as possible. Two Kirschner wires are drilled in to keep the saw on the desired superficial plane. A template for the wires was used as shown here.
The half-moon-shaped polyethylene plateau should just cover the bony tibial plateau, extending precisely to its edge but not past it. The component was available in three lengths: 45, 49 and 53 mm. The runners for the femoral component were available in two lengths, which differ only in the length of the anterior tip.
Trial prostheses and motion testing
Generally the goal of the arthroplasty is to correct varus or valgus deformity of the leg, restoring limb alignment to a physiologic 6° valgus position or directing the mechanical limb axis through the center of the knee joint. With the sled temporarily placed on the femur, we use a trial plateau placed beneath the sled and extend the knee to test the quality of axial correction against the tension of the collateral and cruciate ligaments. Normally the ligaments maintain approximately equal tension in all positions through the range of knee motion. If extension is limited, it can be improved either by using a thinner tibial component or by resecting some additional bone from the opposing anterior surface of the femoral condyle. If flexion is limited or difficult, more bone can be removed from the posterior surface of the condyle and the prosthesis shifted to a somewhat more anterior position. If the knee joint shows increased terminal rotation in extension with external rotation of the tibia, the lateral artificial tibial plateau should be rotated slightly inward toward the anterior eminence. In cases where we were unable to achieve perfect correction of the limb axis, we have accepted small deviations of a few degrees or performed a bicompartmental arthroplasty, resecting some additional bone from the lateral femoral condyle to correct for varus angulation. We do not release the collateral ligaments. Using polyethylene plateaus in 1-mm thickness increments, we can achieve acceptable angular correction without placing too much tension on the medial collateral ligament because it causes pain. At the lateral ligament there are no such problems.
Cementing technique
When the size and placement of the components have been determined, a 4 mm wide groove is made in the tibial plateau with a straight-edge gouge to create a channel for the anchoring tab of the tibial component. Initially the tab was at the center of the plateau. Later we moved the tab to the edge of the intercondylar eminence, where we open the cancellous bone anyway when leveling off the plateau. Based on the results of this study, we moved the tab back to the center, where the greatest loads occur. Additionally, several 2- to 2.5-mm anchoring holes for the bone cement are drilled into the tibial plateau and condyle to reinforce the fixation. The femoral and tibial components should be cemented into place separately. Pressure is placed on the tibial component with a special broad spatula until the component is solidly in place. This appears to be especially important with thinner plateaus.
Additional measures
In patients with flexion contractures, the articular surface of the tibial plateau often shows an excessive posterior downslope, sometimes on both sides. Substance must be removed anteriorly to obtain a horizontal placement of the tibial component and permit full extension of the knee. Following the correction of genu varum or valgum, the previously stretched collateral ligament becomes even more lax. This is relatively unimportant on the lateral side. But a lax medial collateral ligament following the correction of genu valgum should be retightened by advancing its bony attachment on the femur. Bony prominences on the articular margins that might abrade the collateral ligaments should be removed along with spurs projecting into the intercondylar notch that damage the anterior cruciate ligament. There have also been a number of cases where we found it necessary to tailor the patella. We trimmed the overhanging lateral edge of the patella to give her a V-shape. (Fig. 6). In these patients the tibial tuberosity with the tendon of the patella is lateralized and we have to advance it medially. This was seen in patients with decreased acetabular and femoral anteversion (Tönnis, Heinecke1999). The tibial tuberosity can also be transposed 0.5-1 cm proximally to relieve excessive pressure. By using these techniques, we have been able to avoid completely the use of patellar implants.
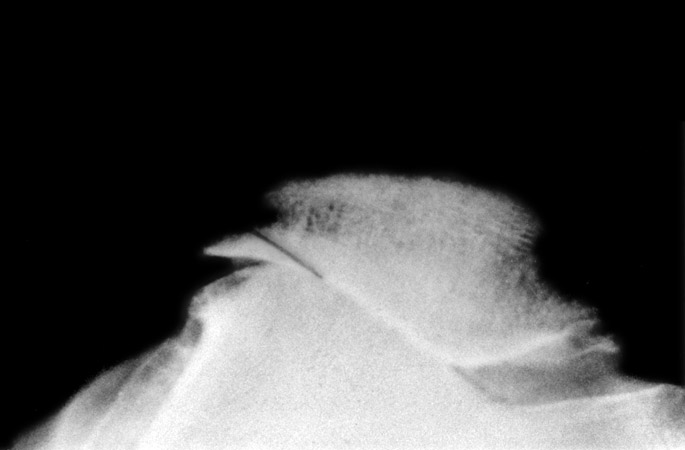 (Fig. 6) Tangential radiograph of the patella shows lateralization, lateral joint space narrowing, and overhang. A line is drawn were we saw off the overhanging part. The position of the patella can be adjusted by medial transposition of the tibial tuberosity.
(Fig. 6) Tangential radiograph of the patella shows lateralization, lateral joint space narrowing, and overhang. A line is drawn were we saw off the overhanging part. The position of the patella can be adjusted by medial transposition of the tibial tuberosity.
Pictures of the operative Technique
In Fig. 7-8 and 9-10 a genu varum is corrected by an unicompartmental prosthesis. In Fig. 11-12 a bicompartmental arthroplasty was necessary to correct the varus deformity. In Fig. 13-14 and 15-16 a moderate and a severe dislocation of the knee were reduced with bicompartmental arthroplasties and achieved an almost normal axis. Fig. 17-18 and 19-20 show a moderate and a severe genu valgum, both corrected by our unicompartmental sledge prosthesis. The lateral collateral ligament almost always can be stretched without difficulties.
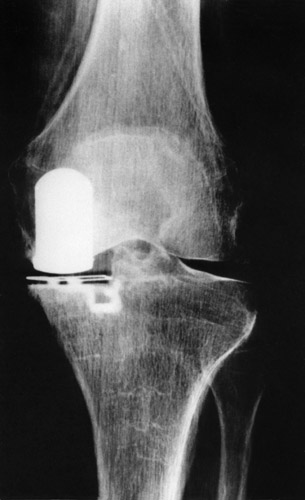 (Fig. 10) The medial collateral ligament could be stretched only to a moderate degree, but to a still quite good result. The lateral view of this joint is seen in Fig. 4.
(Fig. 10) The medial collateral ligament could be stretched only to a moderate degree, but to a still quite good result. The lateral view of this joint is seen in Fig. 4.
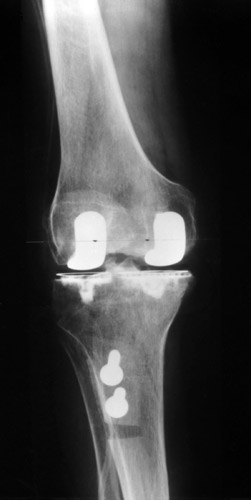 (Fig. 12) The medial collateral ligament was too tight to achieve a normal valgus position. In these cases we take off a superficial portion of the lateral condyle. The tibial tuberosity was taken off for a better access laterally and then fixed again, placing it slightly proximally to increase flexion.
(Fig. 12) The medial collateral ligament was too tight to achieve a normal valgus position. In these cases we take off a superficial portion of the lateral condyle. The tibial tuberosity was taken off for a better access laterally and then fixed again, placing it slightly proximally to increase flexion.
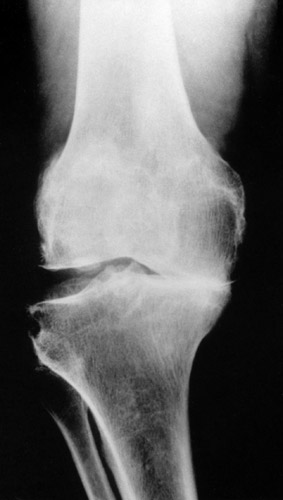
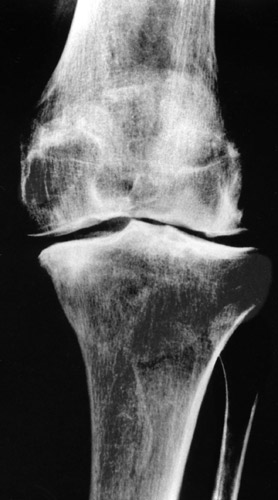 (Fig. 13) Genu varum with a moderate subluxation laterally. The condyles are already deformed and flattened.
(Fig. 13) Genu varum with a moderate subluxation laterally. The condyles are already deformed and flattened.
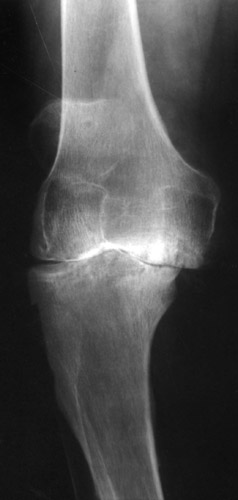
 (Fig. 14) With bicompartmental sledge prostheses and a higher tibial polyethylene plateau medially a quite good axis of the leg could be achieved.
(Fig. 14) With bicompartmental sledge prostheses and a higher tibial polyethylene plateau medially a quite good axis of the leg could be achieved.
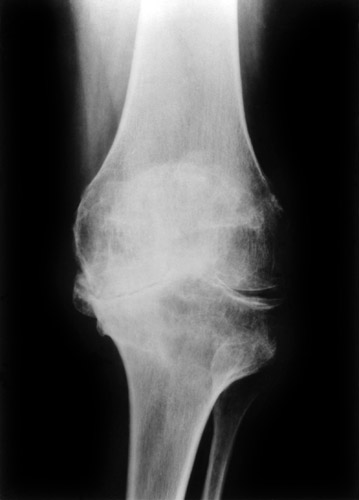
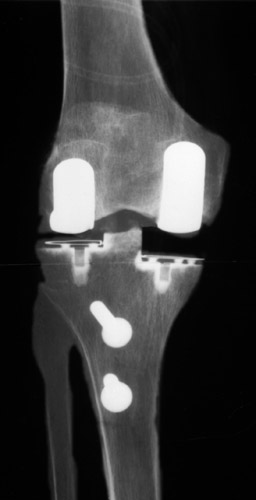
 (Fig. 15) Extreme subluxation of the knee into varus position with severe bone attrition and defomation.
(Fig. 15) Extreme subluxation of the knee into varus position with severe bone attrition and defomation.
 (Fig. 16) The medial collateral ligament allowed a quite good amount of stretching with a high tibial component. Reduction of the subluxation and a fair axis of the leg were achieved by bicompartmental arthroplasty.
(Fig. 16) The medial collateral ligament allowed a quite good amount of stretching with a high tibial component. Reduction of the subluxation and a fair axis of the leg were achieved by bicompartmental arthroplasty.
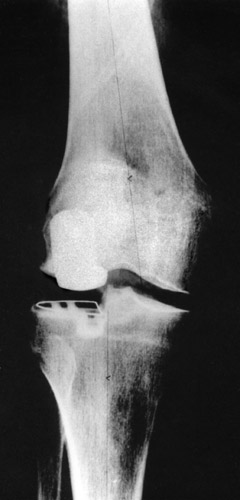 (Fig. 18) With a high tibial polyethylene plateau laterally a normal axis could be achieved. The lateral collateral ligament can be stretched easily to a great degree.
(Fig. 18) With a high tibial polyethylene plateau laterally a normal axis could be achieved. The lateral collateral ligament can be stretched easily to a great degree.
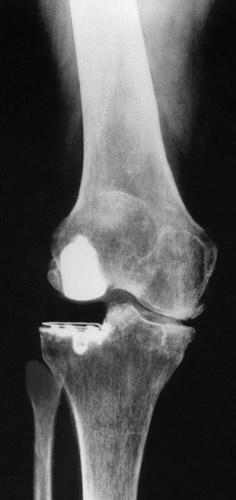 (Fig. 20) With a very high lateral tibial component the axis was corrected, but not completely, to avoid a too high load of the already small medial joint space.
(Fig. 20) With a very high lateral tibial component the axis was corrected, but not completely, to avoid a too high load of the already small medial joint space.
FIRST CLINICAL MATERIAL
We first reexamined patients who had been operated between 1974 and 1985. The clinical material consisted of 424 joints that had been treated with sled prostheses. We personally reexamined 280 patients, and we reviewed 79 from their files; 65 were unavailable for follow-up. Thus, we were able to evaluate 359 arthroplasties (84.7%) after periods of 1 to 11 years, including 19 Marmor prostheses after 10 to 11 years. 227 prostheses had been implanted medially, 32 laterally, and 100 in both compartments, for a total of 309 patients. 39 prostheses were of the first modular type designed by Marmor (1973), and 315 were the Tönnis type of sled prosthesis (1979). Five joints had a Marmor femoral component combined with a Tönnis tibial component.
| Criteria | Joints (No) | Joints (%) | |
|---|---|---|---|
| 1 | narrowing of jointspace | 67 | 18.7 |
| 2 | obliteration of joint space | 57 | 15.9 |
| 3 | minor bone attrition | 31 | 8.6 |
| 4 | moderate bone attrition | 111 | 31.0 |
| 5 | major bone attrition, subluxation | 91 | 25.3 |
| not specified | 2 | 0.6 |
The age at operation was between 36 and 89 years, with an average of 69 years. The severity of osteoarthritis was graded according to joint-space wear using the Ahlbäck (1968) scale (Tab. 1).
RESULTS
Pain
37.9% of the joints were free of pain at follow-up, 37.6% had occasional mild pain, 13.1% had moderate initial pain on walking, and 11.4% had severe pain. The pain was localized to the medial joint space in 27.8% of cases, the lateral joint space in 13.3%, and the patella and its tendinous attachments in 21.2%.
Walking ability
Walking ability was significantly improved. It was unlimited in 46.6% of the cases. The rest showed varying degrees of limitation, but this was due mostly to pain in other joints.
Range of motion
By the time of follow-up, the average range of knee motion had improved from 7.6° of extension loss to 2.6°, and from 100.6° to 107.4° of flexion.
Cruciate and collateral ligament strength
The strength of the collateral ligaments, evaluated by the graded stress testing of medial and lateral joint-space opening immediately after the operation, was significantly increased by the axial correction. Collateral ligament strength decreased slightly over time in joints that were found to have no or only 5° of medial opening, but the joints with greater preoperative opening showed significant, lasting improvement. Unlike some other authors, we found no collateral ligament laxity as a result of the operation. Seven percent of the knee joints showed varying degrees of anterior drawer laxity before the operation. After the operation, only 2.4% of the joints showed the mildest grade of 0.5 cm.
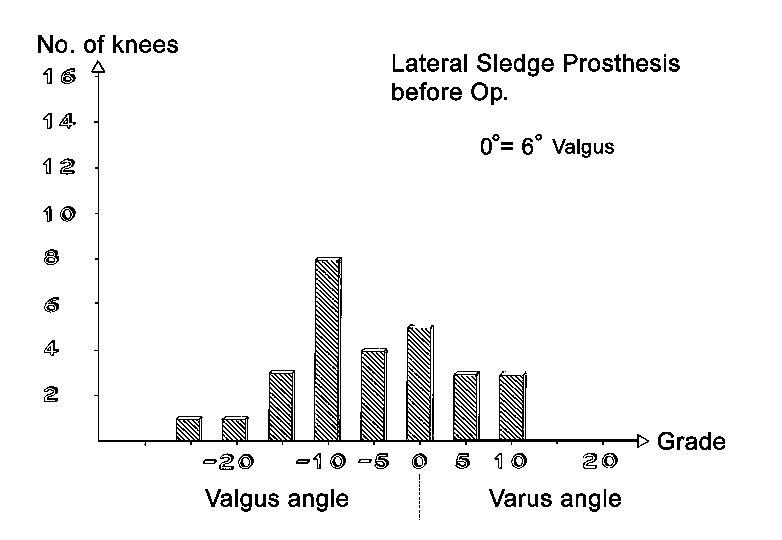
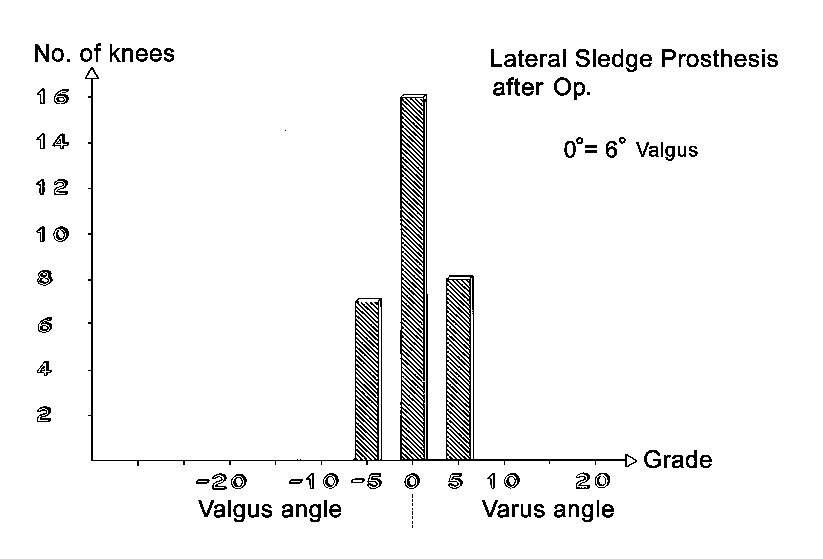
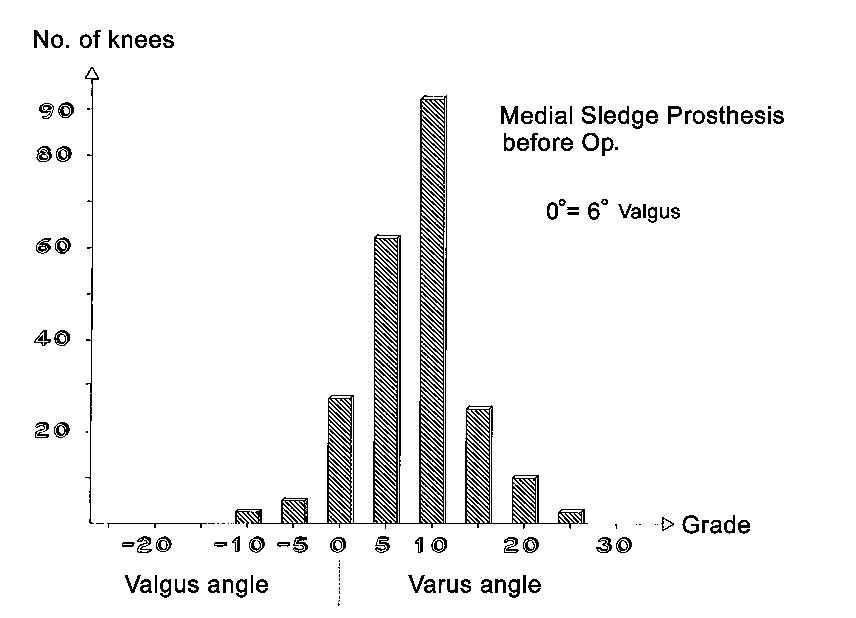
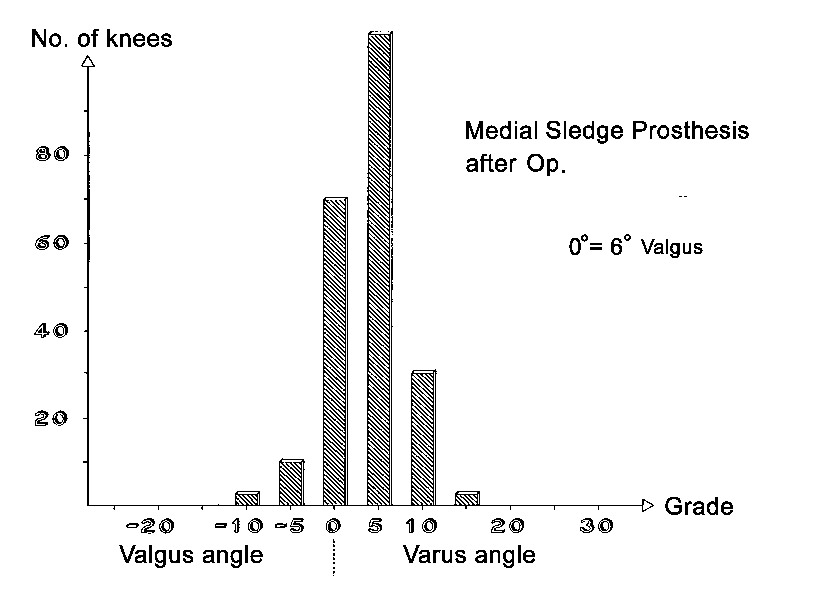
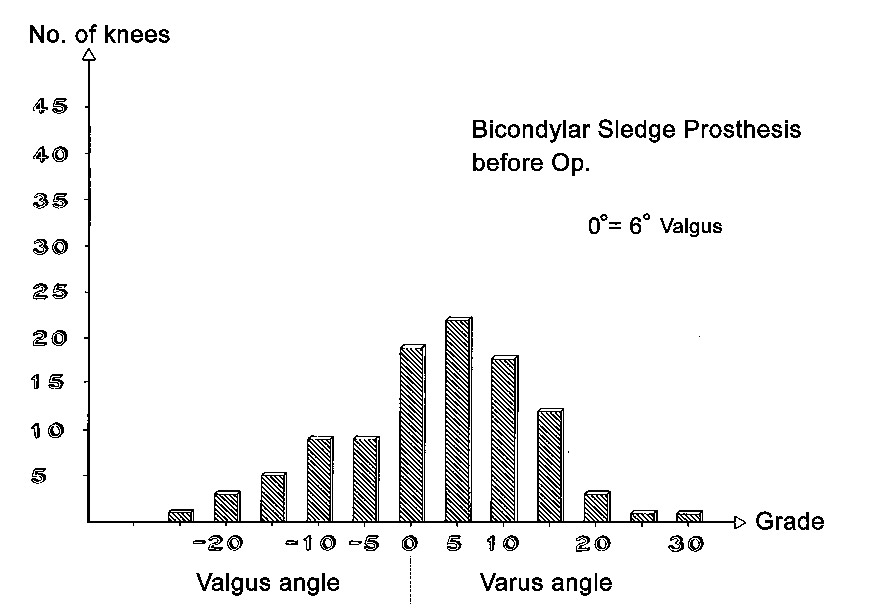
Axial correction
Full-length standing leg radiographs were taken before the operation. The follow-up films consisted of long knee-joint views covering a length of 60 cm. The results in angles are shown in Figs. 21 through 26. Valgus angles were corrected to normal values in almost all cases. The correction of genu varum, unfortunately, is often opposed by a stiff medial collateral ligament. Also, initially we did not have all tibial plateau components available in 1-mm thickness increments. So while we were able to correct more severe degrees of genu varum deviating as much as 27° from the normal 6° valgus, there were significant numbers of patients who were at the 5° level of varus deviation after the operation. Greater deviation was present only in isolated cases. The favorable results were due in large part to the greater varus correction options that are available with the bicompartmental arthroplasty.
Preoperative and postoperative subluxation
Varying degrees of subluxation of the femur were found preoperatively in lateral direction in 0% (n=66) and in medial direction in 16% (n=56), (Figs. 13 and 15). Postoperatively only nine of these joints had a subluxation up to 5 mm and one of 5 mm up to 1/3 the width of the femoral condyle.
Subjective result
67.8% of the patients rated the result of the operation as "very good," 16.5% said they were "reasonably satisfied," 7.7% said the result was "fair" and they were "not entirely satisfied," and 8.0% rated the result as "poor." Nevertheless, 92.6% believed that the decision to have the operation was correct.
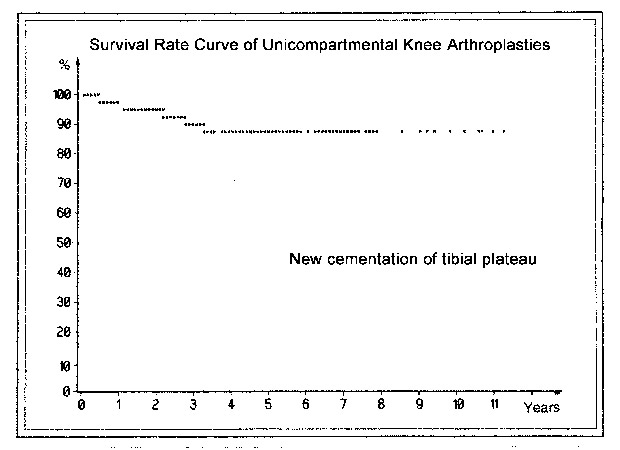
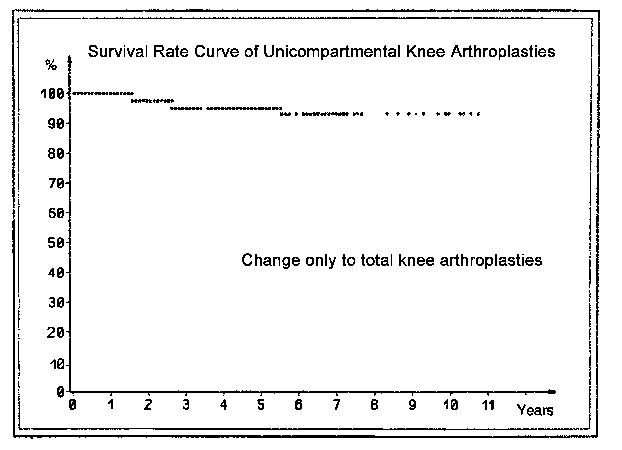
Survival curves
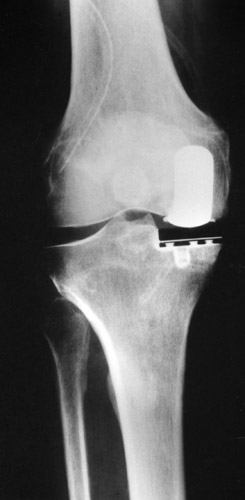
Pain following arthroplasty can have various causes. Overloading of the medial tibial plateau after an inadequate axial correction or complete resection of the subchondral bone plate and trabecular bone layers leads to the appearance of a broad radiolucent line below the tibial component. If the width of this line reaches 2 mm and encompasses the whole undersurface of the polyethylene plateau including the anchoring tab, and if the knee is painful on weight-bearing, we assume that prosthetic loosening has occurred. Kaplan and Meier (1958) introduced the "survival curve" to indicate the success of a prosthesis. The end point may occur due to exchange of the tibial component (Fig. 27) or if there is no prospect for successfully inserting a new tibial plateau and it is necessary to resort to a total knee replacement or arthrodesis (Fig. 28).
In revision cases that required recementing of the tibial plateau, the survival curve fell to just slightly below 90% by the 11th postoperative year. In cases that were changed to a total arthroplasty or arthrodesis, it remained just slightly above 90%. Presumably this can be considered a good result. It is noteworthy that almost all exchange operations were performed during the first 3-4 years. In patients with rheumatoid arthritis or aggressive synovitis 31.3% of the implants, including the sledges, were overgrown and loosened. This did not occur in other patients. Initially, other exchange operations were necessary at an early stage due to technical deficiencies, especially in the patients with Marmor prostheses and a deficient tibial plateau. The revision rate for the Marmor prostheses was 14.6%, compared with 6.3% for our own implants. Taking into account prostheses that are loose by our definition (see above) but have not yet been revised, the rate of successful (nonloosened) sled prostheses in the 11th postoperative year is 82.1%.
Success Depends on Various Factors
Our criteria for failure were a postoperative recurrence of pain, a significant radiolucent line below the plateau component, and the need for reoperation. We investigated these three criteria to determine their dependence on various factors.
Thickness of the tibial component
All of the Marmor prostheses had an initial plateau thickness of 6 mm. The thickness of the Tönnis prosthesis was 4-6 mm in 63.5% of cases and 4-7 mm in 85.1% of cases for both the medial and lateral compartments.
In Table 2 a plateau thickness of 4 mm is associated with a reoperation rate of 24.1%, compared with 11-16% for the 5, 7 and 8-9 mm plateaus and 28.0% for the 10-17 mm plateaus. The 6 mm group as a whole shows a higher loosening rate (28.2%) due to the high percentage of 6 mm Marmor plateaus in that group and is excluded from statistical analysis. When the thin 4 mm plateaus in Table 3 are compared with the overall rates for the 5, 7 and 8-9 mm plateaus, a statistically significant difference is found in the chi-square test. When they are compared with the thickest 10-17 mm plateaus, the chi-square test also shows a p value less than 0.05%, but the number of 10-17 mm plateaus is actually too low for a statistically valid comparison. We also determined confidence intervals, therefore. The curves for the 4 mm and 10-17 mm plateaus do show different peaks for the 5, 7 and 8-9 mm groups, but their bases show slightly too much overlap. It is noteworthy, however, that the distribution ranges of the 5 and 7 mm plateaus do not differ from those of the 8 and 9 mm plateaus. This is interesting when we consider current recommendations that tibial components thinner than 8-9 mm should not be used. The confidence limit was 0.95. The fact that plateau thicknesses of 10--17 mm were associated with the highest loosening rate of 28% supports our belief that tibial components should not be cemented to cancellous bone. Further studies are needed with larger case numbers in order to confirm this, however.
| Height (mm) | 4 | 5 | 6 | 7 | 8-9 | 10-14 |
|---|---|---|---|---|---|---|
| Reoperations necessary (%) | 24.1 | 11.6 | - | 16.1 | 12.3 | 28.0 |
| n = | 72 | 86 | (39) | 62 | 57 | 25 |
Degree of axial correction
Table 3 shows a significant increase in loosening and reoperation rates associated with different degrees of postoperative varus. This increase becomes significant at 7° or more of deviation from the normal value of 6° valgus, doubling to 34% (chi-square test = 0.033). There is only about a 5% overlap between groups 1 + 2 and group 3 with 7° or more of varus, and the confidence limit is 0.95, indicating that the rate of loosening and reoperation increases significantly when there is 7° or more of varus deviation from the normal position of 6° valgus.
| Varusposition in radiography | 0-3° | % | 4-6° | % | 7° and more |
10-14 |
|---|---|---|---|---|---|---|
| No reoperation | 78 | 83.0 | 85 | 82.5 | 33 | 66.0 |
| Reoperation | 16 | 17.0 | 18 | 17.5 | 17 | 34.0 |
The inverse correlation is also interesting. In cases where varus deformity was corrected by more than 10°, the patients no longer had moderate or severe pain, and the reoperation rate was only 4.6%. The case numbers were too small, however, to be statistically significant. The same relationship was found for valgus corrections.
Complication rates of medial, lateral, and bicompartmental prostheses.
Table 4 shows that bicompartmental arthroplasty led to complete relief of pain more often (42.9% of cases) than unicompartmental medial arthroplasty (35.8%). But in degree 2 of pain the medial arthroplasty shows a higher percentage than the bicompartmental (39.9% to 33.7). The reoperation rate was lowest, at 9.4%, for lateral sleds (Table 5). The rate for bicompartmental sleds was 2% lower, at 20.4%, than the rate for medial sleds.
| Compartment (n=351) | medial | % | lateral | % | bilateral | % |
|---|---|---|---|---|---|---|
| n= | 221 | 32 | 98 | |||
| Free of pain | 79 | 35.8 | 12 | 37.5 | 42 | 42.9 |
| Pain at beginning of walking | 88 | 39.8 | 11 | 34.4 | 33 | 33.7 |
| Moderate pain | 33 | 14.9 | 6 | 18.7 | 7 | 7.1 |
| Severe pain | 21 | 9.5 | 3 | 9.4 | 16 | 16.3 |
| Not mentioned | 6 | 2 |
While some authors reported a more frequent need for subsequent surgery on the contralateral joint space, we had only five such cases in a series of 259 unicompartmental arthroplasties: two on the medial side and three on the lateral side. Primary bicompartmental arthroplasty was performed in 100 joints.
Additional results
It was noted at operation whether the anterior cruciate ligament showed moderate or severe fraying or was completely absent. Absence of the anterior cruciate ligament did not adversely affect the outcome, and apparently it is sufficient to stabilize the collateral ligaments in older patients. Faulty positioning of the tibial plateau and sled runner by up to 10° of deviation had no adverse effects, nor did overweight in cases where it was present. The reoperation rate in patients with postoperative stable collateral ligaments was 18.6%. If the joint space showed 6-10° of varus-valgus opening, the reoperation rate increased to 28.6°. Tibial plateaus in sledges with a longer anterior runner loosened 7.1% more often than sleds with the shorter runner in the Tönnis model and 8.5% more in the Marmor prosthesis. The reoperation rate in patients with rheumatoid arthritis and other forms of aggressive synovitis was 31.3%, versus 16.5% in patients with idiopathic osteoarthritis of the knee. A 2-mm radiolucent line was present in 23.3% of patients in the first group, but in only 4.6% of the second group. Therefore we excluded this group of patients later from unicompartmental arthroplasties.
Initially the anchoring tab at the bottom of the tibial component was placed at the center of the plateau and thus at the center of the load-bearing area. Five of these knee joints were available for follow-up. The tibial components showed no loosening and no radiolucent line, whereas the plateaus with a medial tab (Fig. 3) allowed a springy motion of the lateral part of the disk and were more often associated with a 1-mm radiolucent line. This prompted us to change back to a central tab, and we have had very good results with this design over the years. Having the tibial components available in 1-mm thickness increments is important for achieving maximum axial correction without overstretching the collateral ligament.
LATER REINVESTIGATIONS OF OUR TYPE OF PROSTHESIS
Buckup et al. (2001) from our center later conducted a longer-term follow-up study on a single type of implant: the Tönnis medial sled prosthesis. Of the 90 medial prostheses implanted in 1984, 47 (74.6%) were available for follow-up. Twenty-seven patients had died, two could not be located, and 14 did not present for follow-up. The average age at operation was 66.4 years, and the age at follow-up averaged 78.0 years. The follow-up period ranged from 10.8 to 12.7 years, with an average of 11.5 years. The revision rate was 14.9%. Only one case had to be revised to a total knee arthroplasty. Thus the survival rate of the implants, including loose medial implants not yet revised, was 82.1% after 11.5 years. This rate is consistent with the survival curve of the Swedish collective statistics for unicompartmental prostheses (Lindstrand et al. 1991). This series did not include rheumatoid patients.
Later 22 patients of 25 (88.5%) with a lateral prosthesis were reinvestigted after 12 years (Buckup 2005). The height of the tibial plateau ranged from 6 mm (n=8), 7 mm (n=5), 8 mm (n=5) to 11 mm (n=4). Reoperations have not been necessary and significant radiolucent lines of 2 mm were not found. 10 patients rated the result as "very good", 3 as "good", one as "fair" and one as "poor".
Kisslinger et al. (2001) applied the same principle of preserving the subchondral bone plate and using a thinner polyethylene plateau combined with a sled prosthesis. Initially they also used the St. Georg sledge and Endo 2 model before changing to the Tönnis prosthesis. Wessinghage made some additional modifications to the device (1990, 1991). A total of 261 unicompartmental knee arthroplasties (98.5%) were evaluated in 229 patients. Survival with aseptic failure as the endpoint was 84.9% after 10 years and 74.4% after 20 years. The 10-year survival rate in this series almost precisely matches the rate at our center. Tibial components with a thickness of 6 mm were used in 47.1% (n = 123) of the joints, components 7 mm thick in 25.6% (n = 67), and components thicker than 7 mm in 20.3% (n = 63). Information was missing in 8 joints (3.1%).
DISCUSSION
Based on our experience, we do not agree with rejecting the use of sledge prostheses or limiting their use exclusively to medial osteoarthritis or moderate grades of osteoarthritis. Over time, in fact, we have come to treat increasingly severe grades of osteoarthritis with unicompartmental prostheses and with bicompartmental replacements instead of a total prostheses, partially with involving release of the tibial tuberosity (Fig. 29-33).
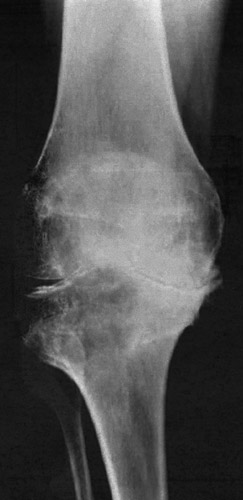 (Fig. 29) Severe degree of osteoarthrosis. Even in this case a bicompartmental arthroplasty was possible.
(Fig. 29) Severe degree of osteoarthrosis. Even in this case a bicompartmental arthroplasty was possible.
Regarding the wear of our prostheses, we note that despite the gentle curve of the articular surface of the sled runner, the runner naturally only makes point contact with the opposing polyethylene plateau. As wear occurs, the contact area between the components increases. But our survival curves demonstrate, that our tibial plateaus undergo very little change over a long period of time. Of course, most of our patients were elderly, and it is conceivable that greater wear will occur in younger individuals. But our implantation technique leaves open the option of exchanging the plateau since it requires very little resection of the subchondral bone.
Advantages of the Prosthesis and Technique
- This arthroplasty largely preserves the knee joint including all its ligaments and the proprioceptive endings in the ligaments and infrapatellar fat pad. Furthermore, the patella has only little contact to the metal of the sledges compared to total knee arthroplasties.
- The use of thinner components preserves the weight-bearing surfaces of the femoral condyles and tibial plateau. This reduces the risk of loosening. Fractures of the metal sledge have not been observed, and deformation of the tibial polyethylene plateau has generally been seen only in the early Marmor tibial components that did not have an anchoring tab.
- If loosening occurs, it is usually possible to replace and recement the component without converting to a total knee arthroplasty.
- Bicompartmental arthroplasty can be done for more severe grades of bilateral osteoarthritis and axial deformities and yields good results. We had only five cases in 359 unicompartmental arthroplasties that required subsequent treatment of the contralateral joint space.
- Osteoarthritis treatment does not require the use of patellar implants. Complaints relating to a lateralized patella can be adequately managed by V-shaped trimming of a deformed patella and by medial advancement of the tibial tuberosity.
- Tibial plateau heights in 1-mm increments allow for gentle stretching of the collateral ligaments for axial limb correction and avoids overstretching.
- The posterior surface of the femoral condyle can be trimmed to relieve excessive tension on the extensor muscles. This avoids patellar tendinopathy and limitation of flexion.
References
- Ahlbäck S (1968) Osteoarthrosis of the knee. A radiographic investigation. Acta radiol Suppl 277
- Bartel DL, Bicknell V, Ithaka MS, Wright TM (1986) The effect of conformity and plastic thickness on contact stresses in metal-backed plastic implants. J. Bone Joint Surg 68 A:1041-1051
- Bourme RB, Finlay JB, Papadopoulos P, Andreae P (1984) The effect of medial meniscectomy on strain distribution in the proximal part of the tibia. J Bone Joint Surg 66 A:1431-1437
- Buckup K, Runge J, Katthagen BD (2001) The long-term results of unicompartmental knee arthroplasty. ISAKOS–Congress, Montreux, Schweiz, May 2001
- Buckup K (2005) Die unicondyläre Schlittenprothese. Pro & Contra. Steinkopff, Darmstadt, pp 78-87
- Engelbrecht E (1971) Die Schlittenprothese, eine Teilprothese bei Zerstörungen im Kniegelenk Chirurg 42:510-514
- Finlay JB, Bourme RB, Kraemer WJ, Morroz TK, Rorabeck CH (1989) Stiffness of bone underlying in the tibial plateaus of osteoarthritic and normal knees. Clin Orthop 247:193-201
- Goldstein SA, Wilson DL, Sonstegard DA, Matthew LS (1983) The mechanical properties of human tibial trabecular bone as a function of metaphyseal location. J Biomech 16 (129):965-969
- Harada Y, Wevers HW, Cocke TD (1988) Distribution of bone strength in the proximal tibia. J Arthroplasty 3 (2):167-175
- Hoffmann P (1985) Optimierung der Materialstärke des metal-backed tibiaplateaus unter Berücksichtigung der Hertzschen Pressungstheorie. Diplomarbeit der Fachhochschule München. Fachbereich 06, Feinwerk- und Mikrotechnik, München
- Hvid I, Soondergaard B, Larsan (1983) Compressive strength of tibial cancellous bone. Instrom osteopenetrometer measurements in an autopsy material. Acta Orthop Scand 54(6):819-825
- Hvid I, Hansen SL (1985) Subchondral bone strength in arthrosis. Cadaver studies of tibial condyles. Acta Orthop Scand (1):464-472
- Hvid I, Jensen J, Nielsen S (1985) Contribution of the cortex to epiphyseal strength. Acta Orthop Scand 56(3):256-259
- Hvid I, Jensen J, Nielsen S (1986) Bone strength measurements at the proximal tibia. Penetration tests and epiphyseal compressive strength. Int Orthop 10(4):271-275
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J. American Statistical Association 53:457-481
- Kilgus DJ, Moreland JR, Finerman GA, Funahashi TT, Tipton JS (1991) Catastrophic wear of tibial polyethylene inserts. Clin Orthop 273:223-231
- Kißlinger E, Jüsten HP, Wessinghage D (2001) Besser als ihr Ruf ? 5-20 Jahresergebnisse mit Unicompartment-Knieglenksendoprothesen bei medialer Gonarthrose. Z Orthop 139:97-101
- Lindstrand A, Stenström A, Lewold S (1992) Multicenter study of unicompartmental knee revision. PCA, Marmor and St Georg compared in 3.777 cases of arthrosis. Acta Orthop Scand 63:256-259
- Little RB, Wewers HW, Siu D, Cooke TD (1986) A three dimensional element analysis of the upper tibia. Biomech Eng 108 (2):111-119
- Marmor L (1973) The modular knee. Clin Orthop 94:242-248
- Marmor L (1984) Lateral compartment arthroplasty of the knee. Clin Orthop 186:115-121
- Noble J, Alexander K (1985) Studies of tibial subchondral bone density and its significance. J Bone Joint Surg 67 A:293-302
- Odgaard A, Hvid I, Linde F (1989) Compressive axial strain distributions in cancellous bone specimens. J. Biomech (United States) 22(8-9):829-835
- Tönnis D (1979) Eine abgeänderte Schlittenprothese für den Aufsitz auf Kortikalisfläche. Z Orthop 117:833-836
- Tönnis D (1985) Die Schlittenprothese (Modell nach Tönnis), Implantationstechnik, Indikation, Ergebnisse. Med Orthop Tech 105:318-321
- Tönnis D (1985) Die Schlittenprothese (Modell nach Tönnis), Implantationstechnik, Indikation, Ergebnisse. Med Orthop Tech 105:318-321 137, Teil 1: Statistik und klinische Folgen: 153-159, Teil 2: Ätiologie, Diagnostik und Therapie: 160-167
- Tönnis D, Heinecke A (1999) Acetabular and femoral anteversion: Relationship with Osteoarthritis of the hip. Current Concept Review. J Bone Joint Surg 81 A:1747-1770
- Vasu R, Carter DR, Schurmann DJ, Beaupre GS (1986) Epiphyseal based designs for tibial plateau components- Stress analysis in the frontal plane. J Biomech (United States) 19:647-662
- Wessinghage D (1990) Der Gelenkflächen-Teilersatz des Kniegelenkes durch eine verbesserte Schlittenprothese nach Wessinghage. !. Teil. Akt Rheumatol 15:190-194
- Wessinghage D (1991) Der Gelenkflächen-Teilersatz des Kniegelenkes durch eine verbesserte Schlittenprothese nach Wessinghage. 2. Teil. Akt Rheumatol 16:73-77
- Zysset PK (1994) Morphology-mechanical property relations in trabecular bone of the osteoarthritic proximal tibia. J Arthroplasty (2):203-216
| Download | |
|---|---|
| Uni- and bicompartmental arthroplasties with minimal resection of the tibial subchondral bone plate. Long term results related to various factors |
PDF (2.8MB) |